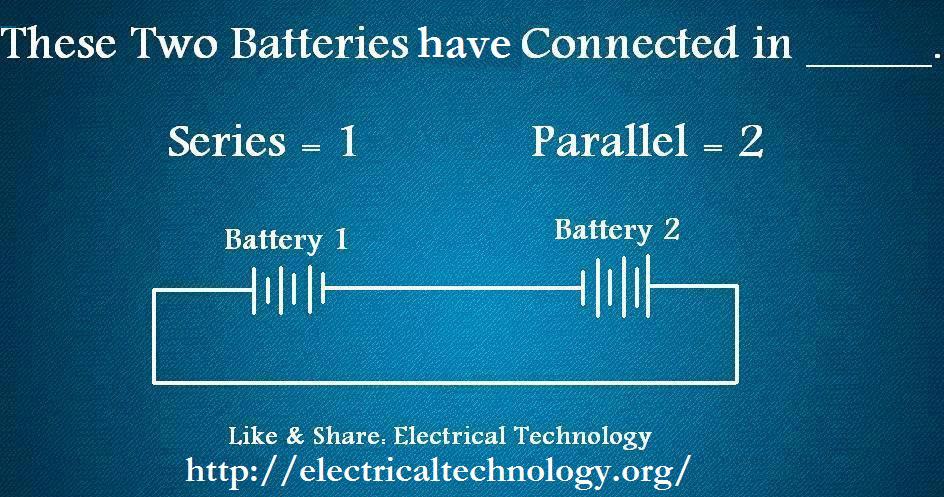

As we can see that Positive connected to positive terminal and Negative connected to Negative Terminal. So the batteries configuration in Parallel.How can we connect a load in this configuration, it is shown in the figure below.

2. In Ideal case, the Charging current for 200Ah battery would be _________ ?
Answer: 4. 20 A.
Explanation:
Charging current should be 10% of the Ah (Ampere hour) rating of battery.
Therefore, Charging current for 120Ah battery would be = 200Ah x (10/100) = 20A.
Note: This is for Ideal case only…for real case. just check MCQs # 3.
3. In Real case, the Charging current for 200Ah battery would be _________ ?
Answer: 1. 20-22A
Explanation:
Charging current should be 10% of the Ah (Ampere hour) rating of battery.
Therefore, Charging current for 120Ah battery would be = 200Ah x (10/100) = 20A
But due to losses, the charging current for 200Ah battery should be 20-22A.
4. In Ideal case, the Charging Time for 200Ah battery would be _________ ?
Answer: 2. 10 hours
Explanation:
Charging current should be 10% of the Ah (Ampere hour) rating of battery.
Therefore, Charging current for 120Ah battery would be = 200Ah x (10/100) = 20A
Hence, Charging Time for 200 Ah battery = Ah rating of battery / Charging Current
= 200Ah/20 = 10 hours.
Note: This is for Ideal case only…for real case. just check MCQs # 5.
5. In Real case, the Charging Time for 200Ah battery would be _________ ?
Answer: 3. 11 hours
Explanation:
Suppose for 200 Ah battery,
First of all, we will calculate charging current for 200 Ah battery. As we know that charging current should be 10% of the Ah rating of battery.
So charging current for 200Ah Battery = 200 x (10/100) = 20 Amperes.
But due to the losses, we can take 20-22 Amperes for charging purpose.
suppose we took 22 Amp for charging purpose,
Then charging time for 200Ah battery = 200 / 22 = 9.09 Hrs.
But this was an ideal case…
Practically, this is noted that 40% of losses ( in case of battery charging)
Then 200 x (40 / 100) = 80 …..(200Ah x 40% of losses)
Therefore, 200 + 80 = 280 Ah ( 200 Ah + Losses)
Now Charging Time of battery = Ah/Charging Current
280 / 22 = 12.72 or 12.5 Hrs ( in real case)
Therefore, a 200Ah battery would take 12 Hrs for completely charging (with 22A charging current).
6 One (1) Ah = ________?
Answer: 4. 3600C
Explanation:
1Ah = (1A) x (3600s) = (C/s) x (3600s) = 3600 C.
∴ A (One Ampere) = One Coulomb per second = C/s
7. The commercial lead acid cell has 13 plates. The number of positive plates would be_______.
Answer: 1. 6
The number of negative plates in a lead acid cell is one more than the number of positive plates ; the outside plates being negative. So the number of positive plates would be 6.
8. The commercial lead acid cell has 15 plates. The number of negative plates would be_______.
Answer: 3. 8
Explanation:
The number of negative plates in a lead acid cell is one more than the number of positive plates ; the outside plates being negative. So the number of negative plates would be 8.
9. A lead acid cell has 15 plates. In absence of manufacturer’s data [nameplate], the charging current should be________.
Answer. 4. 7A
Explanation:
The charging current for battery should be 1A for every positive plate of a single cell. Also we know that The number of negative plates in a lead acid cell is one more than the number of positive plates; the outside plates being negative. therefore, the number of Negative and Positive plates wold be 8 and 7 respectively. thus, the charging current for this battery would be 7A.
10. A Battery is a series or parallel combination of electrolytic cells.
Answer: ( 1 )
An electrolyte cell consists of a positive electrode and a negative electrode separated from each other by an electrolyte. The electrolyte can be concentrated aqueous solutions like acids, alkalis or salts, or ionic conductors like organic salt solutions, polymers, ceramics etc. The electrolyte is a good conductor of ions, but a bad conductor of electrons. Two or more such cells connected together in series or in a series-parallel array forms an assembly called Battery.
11. In a single cell, the two electrodes are separated from each other by——
Answer: ( 1 )
In a single electrolytic cell, the positive electrode and negative electrode have minimum distance between them, about 1mm, so that the internal resistance is as low as possible. Typical value of this resistance is of the order of mill-ohms, so that voltage drops between the electrodes is minimum when drawing huge amount of current
12. Standard open circuit voltage for Lead-acid battery at standard conditions is——-
Answer: ( 2 )
The net voltage or voltage difference between potentials of the positive and negative electrodes of a electrolyte cell defines the open circuit voltage at standard conditions. During discharge of the battery, the negative electrode undergoes oxidation while the positive electrode undergoes reduction as given below:

Thus, open circuit voltage at standard conditions is: V o = E o1 -E 02 = +1.690 – (-0.358) Volts = +2.048 Volts
13. In a primary cell, one of the electrodes is usually used away after certain time with chemical action.
Answer: ( 1 )
Consider a primary cell having Zinc electrode as negative electrode and Carbon electrode as negative electrode and Sulfuric acid as the electrolyte. Movement of electrons from negative electrode to the positive electrode constitutes current flow through the load. The chemical reaction that takes place ensures that the positive ions in the Zinc electrode combines with the negative ions from the Sulfuric acid, forming Zinc Sulfate.
Thus, as the reaction proceeds further, Zinc gradually dissolves into the solution, causing the cell to be discharged. This proves that in a primary cell, one of the electrodes is used away with the chemical action
14. In a secondary cell, electric current is made to flow in opposite direction than usual to achieve charging of the cell.
Answer: ( 1 )
Consider a 12-Volt Lead-Acid battery having fully charged terminal voltage of 12.6 Volts. When the battery is supplied with an external voltage greater than the terminal voltage, smooth charging is ensured.
As electricity passes through the electrolyte solution, the Lead Sulfate at the electrodes converts back to its original substance, i.e. anode or positive plate of Lead Dioxide and Cathode or negative plate of Sponge Lead.
15. A primary dry cell is called so because—–
Answer: ( 2 )
A primary dry cell, also known as Leclanche cell, consists of a moist paste as its electrolyte. The paste is usually a mixture of substances like ammonium chloride, manganese dioxide, powdered coke, graphite and water. The paste is contained in a Zinc container, which acts as the cathode or the negative electrode. The container is lined with a non-conducting material to separate Zinc from the paste.
16. Nickel-Cadmium batteries are preferred more than Lead-Acid batteries in military applications because——–
Answer: ( 4 )
Nickel Cadmium cells contain Cadmium Hydroxide as cathode, Nickel Hydroxide as anode and Potassium Hydroxide and water as electrolyte. Though Nickel-Cadmium batteries are comparable with Lead-Acid batteries at normal discharge rates, at higher discharge rates, the amount of power delivered is higher in the former. Also, Ni-Cd batteries show superiority over Lead-Acid batteries in terms of faster charging rate, less time to charge, longer storage period and longer intervals of charging and discharging.
17. Number of cells connected in series provide a —–
Answer: ( 2 )
Consider the below series combination of four cells.
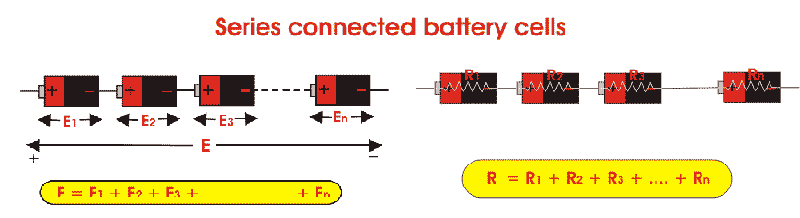
Figure 1: Series Connected Cells
Notice that the negative electrode of each cell is connected to the positive electrode of its adjacent cell. If voltage across each cell is V volts, the total voltage across the loop will be determined using Kirchhoff’s law as sum of the individual voltages across each cell. Hence total voltage will be 4V volts. This shows that cells connected in series provides a higher voltage.
18. Number of cells connected in parallel provide a——-
Answer: ( 1 )
Consider the below parallel combination of four cells.

Figure 2: Parallel Connected Cells
Note that positive electrodes of all the cells are connected in series with each other and the negative electrodes are connected in series with each other. This ensures that voltage across the combination is same as the voltage across a single cell, i.e. V volts. However, the total current flowing would be sum of the individual currents flowing through each cell. Thus, cells connected in parallel constitute flow of higher current.
19. Specific Gravity of a electrolyte in a single cell or a battery is always—–
Answer: ( 2 )
Specific Gravity of a substance is defined as the ratio of its weight compared to the weight of same amount of pure water. This term is used to determine the amount of active ingredient in an electrolyte, to ensure smooth operation of the cell. Since the electrolyte is a solution of water, the amount of active ingredient cannot be measured directly.
Any substance which floats on water will have specific gravity less than that of pure water, i.e. less than 1.0. Since the active ingredient used must sink or dissolve in water, the specific gravity is usually greater than that of pure water, i.e. greater than 1.0.
20. Storage batteries are rated according to ——–
Answer: ( 3 )
Theoretically, capacity or rating of a storage battery is given in terms of the total amount of current which flows through the battery, at a given amount of time and is a constant value. However, practically the rating depends upon the rate of discharge and the ambient temperature. Higher the rate of discharge, lesser will be the capacity.
The discharge occurs till the voltage per cell reduces to 1.7 volts, especially for Lead-Acid batteries. For a storage battery rated 100 Ampere-Hours at 5-Hour discharge rate, if the latter is decreased to 30-minute rate, the capacity delivered will be 1/3 rd of the given nominal capacity. If the discharge rate is increased to 10-Hour rate, the capacity delivered will increase by 20% than the nominal value.
Also, optimal battery performance is achieved in the working temperature range from 20 degree Celsius to 30 degree Celsius. According to few practical tests, discharge rate of battery doubles with every 10-degree Celsius rise in temperature. Though at higher temperature, the rate of occurrence of chemical reactions will be faster, the life of the battery will be reduced.
Related MCQS with Explanatory Answers:
Batteries Related Posts: